Summary
The original article was written in 2021. This is a 2024 summary / rewrite.
I never finished writing the original G-orbital blog post and I’m not sure where I was trying to go with it originally.
I learned in high school chemistry about VSEPR theory and some behavior of electrons in the context of chemistry (well as much as a high schooler could understand). They showed these cool images of atomic orbitals. Here’s an image from wikipedia on the hydrogen orbital.
The colors of the orbitals correspond to some kind of intensity, in this case probability of the electron being in that position, which can also be viewed in 3 dimensions.

It turns out that the geometry of the orbitals is defined by some parametric equations that correspond to vibrations on a sphere. The topic that studies this is called spherical harmonics. I still don’t really understand its derivation.
I wanted to see if it was possible to generate renderings of the different atomic orbitals purely from formulas. Additionally, I wanted to do this in GLSL so I could run it in the browser.
I think that, when I did this 3 years ago, I think I rendered the J orbitals and not the G orbitals since that was already done before. And with some added unnecessary transforms to each orbital to make some cool transitions…
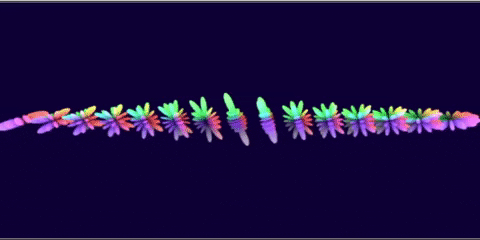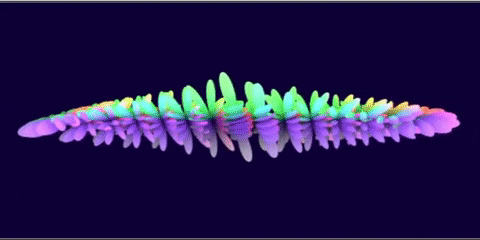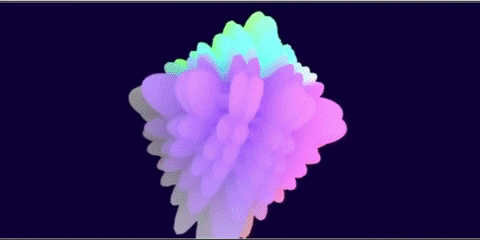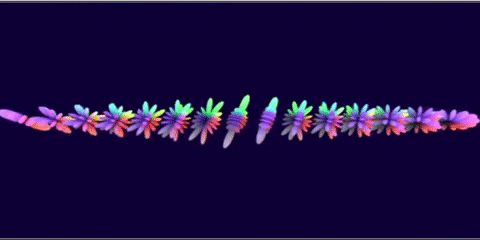
The G-orbital isn’t actually used in practice since it would require elements like Ununennium with an isotope that lasts feasibly long enough to be declared as a discovered element.
 glsl
glsl